For years, many women have been told their stomach pain was “just stress” or “just hormones.” Now, research from the US is showing that hormones like oestrogen don’t simply change mood or fertility. They interact directly with cells in the gut, tweak pain signals, and can turn everyday digestion into a source of chronic discomfort.
When your cycle and your stomach seem to talk to each other
Globally, around one in ten people lives with irritable bowel syndrome (IBS): cramps, bloating, diarrhoea, constipation, or all of the above. Women are affected far more often than men, making up roughly two-thirds of cases.
Many of them share the same story. Medical tests come back normal. Scans show nothing. Standard treatments help a bit, then stop working. And the pain keeps circling back, especially around menstruation, pregnancy, or perimenopause.
IBS pain may be invisible on a scan, but it can be intense enough to disrupt sleep, work and social life.
For a long time, the hormonal pattern was just an observation. Symptoms got worse at certain phases of the cycle, then eased again. Doctors noted it, but could not explain why. That gap has pushed researchers to look closely at how sex hormones interact with the gut’s own nervous system.
Inside the gut: an unexpected hormone–nerve conversation
The new work, led by physiologist Holly Ingraham’s team at the University of California, San Francisco, zeroes in on an intricate chain reaction in the colon.
Not the usual suspects: beyond serotonin alone
Scientists first tested a likely candidate: enterochromaffin cells. These cells line the gut and produce about 90% of the body’s serotonin, a key messenger for both mood and pain. The idea was simple: if oestrogen could switch these cells on directly, it might explain the flare-ups.
That theory did not hold. The cells didn’t appear to respond to oestrogen in the way researchers expected. So the team looked elsewhere in the intestinal lining.
The key role of L cells and the peptide PYY
The breakthrough came with a rarer group of cells called L cells, tucked into the mucosa of the colon. These cells are better known for their role in appetite and blood sugar regulation.
Ingraham’s group found that L cells carry receptors for oestrogen. When they are exposed to the hormone, they release a molecule called peptide YY (PYY). That molecule, in turn, acts on nearby enterochromaffin cells.
Oestrogen doesn’t cause pain directly. It primes a cascade: L cells release PYY, neighbouring cells release serotonin, and the gut’s pain circuitry becomes more reactive.
Once serotonin is released, nerve endings in the gut wall fire more easily. Signals travel along the gut–brain axis, a dense network of nerves and pathways linking the intestines to the central nervous system. The result: sensations that might have passed unnoticed now feel like cramping, stabbing or burning pain.
Why this helps explain female IBS
This chain of events gives a clearer biological basis for sex differences in IBS. Women live with fluctuating oestrogen levels from puberty through menopause. Those rises and falls can repeatedly tweak sensitivity in the gut.
- Before a period, hormonal swings can heighten gut reactivity, making usual bloating suddenly painful.
- During pregnancy, higher oestrogen levels may amplify existing IBS symptoms for some women.
- Hormonal contraception or HRT can, in certain cases, alter digestive comfort, for better or worse.
Instead of treating women’s IBS as a vague, stress-driven problem, the research points to a specific pathway: oestrogen → L cells → PYY → serotonin → pain signalling.
For patients, that pathway is a message: the pain is not “in your head”; it is wired into the biology of your gut.
Food, microbiome and hormones: a three-way interaction
The story does not stop with hormones and nerve cells. Food and gut bacteria join the conversation too.
FODMAPs and the microbiome’s chemical signals
Many people with IBS are advised to follow a low-FODMAP diet. FODMAPs are fermentable carbohydrates found in foods like onions, wheat, dairy and certain fruits. They are poorly absorbed in the small intestine and end up in the colon, where gut microbes feast on them.
As bacteria break these sugars down, they produce short-chain fatty acids. In the new research, these fatty acids appear to activate a receptor called OLFR78 on L cells. Once again, L cells respond by releasing PYY, setting off the same serotonin-driven sensitivity cascade.
Hormones and diet can converge on the same cells. Oestrogen and fermentable sugars both nudge L cells into releasing more PYY.
This helps explain why some people experience explosive symptoms after a seemingly modest serving of pasta, garlic or apples. In women, especially during hormone peaks, that response may be even stronger.
Rethinking treatment: from broad painkillers to targeted strategies
Most current IBS treatments try to manage symptoms: antispasmodics for cramps, laxatives or anti-diarrhoeals for bowel changes, and antidepressants to dull pain signalling. These can help, but they often miss the root mechanism and can cause side effects.
With L cells, PYY and OLFR78 on the map, researchers are considering more precise options:
| Target | Potential strategy | Possible benefit |
|---|---|---|
| L cells | Reduce overactivation without shutting them down completely | Lower pain sensitivity while keeping appetite and glucose control intact |
| PYY signalling | Modulate how strongly PYY talks to neighbouring cells | Interrupt the pain cascade early on |
| OLFR78 receptors | Limit the response to microbe-made fatty acids | Blunt food-triggered flares tied to fermentation |
| Diet and microbiome | Tailor FODMAP restriction and support diverse gut bacteria | Reduce triggering fermentation patterns without extreme diets |
One major concern is balance. These same pathways control satiety, blood sugar and vascular function. A drug that silences them too forcefully could disturb weight regulation or cardiovascular health. Future therapies are likely to be gentler, nudging responses rather than blocking them outright.
What this means for someone living with hormone-linked gut pain
For a woman tracking her IBS in real life, the research suggests practical questions to bring to a GP or specialist.
- Do flare-ups track closely with the menstrual cycle, contraceptive use or hormone therapy?
- Are certain high-FODMAP meals consistently tied to severe pain, even when portion sizes are small?
- Does stress make symptoms worse mainly during particular hormonal phases?
Keeping a three-month diary that notes cycle days, foods, stress levels and pain intensity can reveal patterns. That record can support a more tailored plan, which might combine a time-limited low-FODMAP trial, psychological support such as gut-directed hypnotherapy, and a review of hormone-based medications.
Terms that help make sense of the science
Some of the jargon around this topic can feel impenetrable. A few key terms make it easier to follow the conversation with clinicians:
- L cells: rare hormone-producing cells in the gut lining that respond to nutrients and hormones.
- Peptide YY (PYY): a hormone released after eating, involved in satiety, now linked to pain sensitivity.
- Enterochromaffin cells: gut cells that release serotonin and pass signals to nerve endings.
- Serotonin: well known in mood regulation, but in the gut it acts as a key driver of motility and pain signalling.
- OLFR78: a receptor on L cells that detects substances made by gut bacteria.
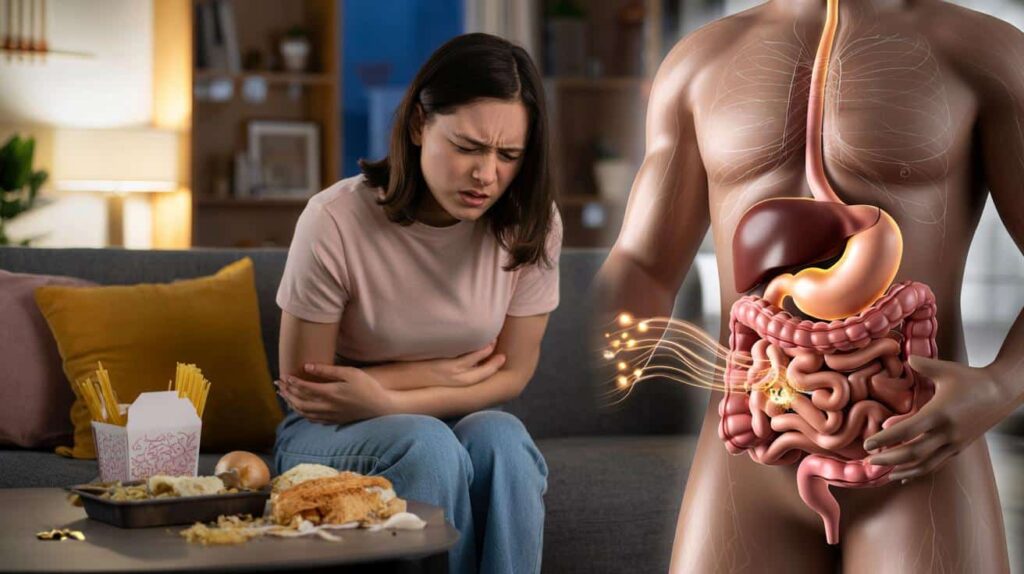
Picture an evening scenario. A woman in her late 20s, a few days before her period, eats a garlic-heavy takeaway with wheat noodles. Her hormones are already priming L cells to respond more strongly. Fermentable carbs reach the colon, microbes generate short-chain fatty acids, OLFR78 lights up, PYY surges, serotonin follows – and within hours, she is curled on the sofa with cramps that no scan will ever show.
Research into hormonal amplification of gut pain does not make that evening any less painful. It does, though, turn a vague mystery into a concrete biological story, and that shift can guide better questions, better trials and, gradually, more targeted options for the millions who feel every twist of this hidden conversation between hormones and the digestive tract.





